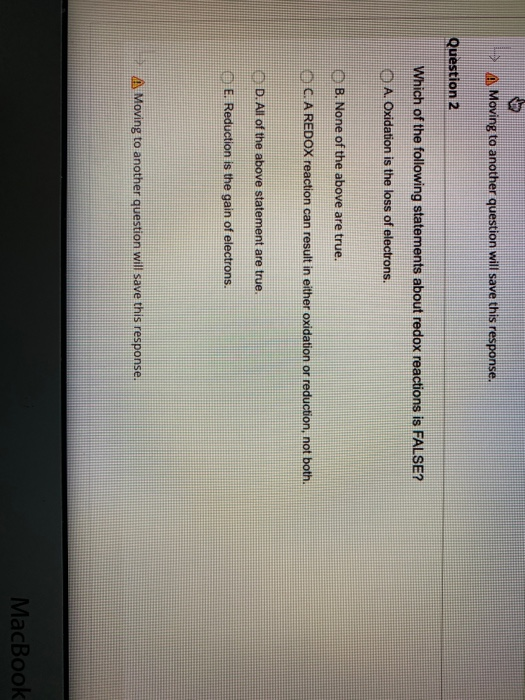
Which of the following statements about redox reactions in biochemistry is FALSE. 10 Which of the following statements about redox reactions is FALSE.
Solved Moving To Another Question Will Save This Response Chegg Com
B Reduction is the gain of electrons.

. A Oxidation is the loss of electrons. School University of Guelph. Pages 13 This preview shows page 8 - 12 out of 13 pages.
250 TOP MCQs on Biological Oxidation-Reduction Reactions and Answers. OTHER SETS BY THIS CREATOR. An oxidizing agent gains.
B A reaction can result in either oxidation or reduction not both. A O 2 is the strongest oxidant commonly encountered in biochemical processes. Which of the following statements about redox reactions in biochemistry is FALSE.
E All of the above statement are true. E All of the above statement are true. Course Title BIOC 2580.
Oxidation and reduction a. C FAD is a stronger oxidizing agent than NAD. The electrode in the left half-cell is the cathode because oxidation occurs here.
D Oxidation is the loss of electrons. A A reaction involving elemental oxygen is a redox reaction. OxygenH 2 O redox pair has the highest redox potential.
Accompany all chemical changes c. Ma 162 Unit 2 Important Formulas. NADHNAD redox pair has the least redox potential.
Describe the loss and gain of electrons respectively d. Biochemistry Multiple Choice Questions on Biological Oxidation-Reduction Reactions. C A reaction can result in either oxidation or reduction not both.
Which of the following statements about redox potential is false. Which of the following statements is are true. The standard cell potential is positive so the reaction is spontaneous as written.
An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule atom or ion changes by gaining or losing an electron. D A reaction involving elemental oxygen is a redox reaction. A reducing agent reductant causes another substance to be reduced.
Result in a change in the oxidation states of the species involved e. B Most biological oxidations involve reactions between an organic substrate and O 2. Which of the following statements given about redox reactions is FALSE.
Cannot occur independently of each other b. A b An oxidizing agent contains an element whose oxidation state decreases in a redox reaction and is reduced. Which of the following statements about redox.
An oxidizing agent oxidant causes another substance to be oxidized. C Reduction is the gain of electrons. An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species.
Write true if the statement is correct and if false underline the wrong word or phrase and write the correct word to make the statement true.
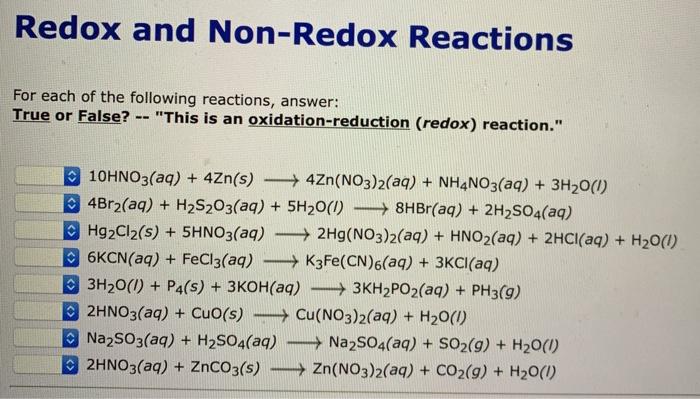
Solved Redox And Non Redox Reactions For Each Of The Chegg Com

Solved Q16 Classify Each Of The Following Statements As True Or False Oxidation Takes Place At Anode T Er Reduction Takes Place At Cathode T74e Electrolysis Is A Spontaneous Reaction False The Oxidation Number

Which Statement Is True Regarding Redox Reaction Socratic

Solved Classify The Statements About Redox Reactions As True Chegg Com
0 Comments